Genomics involves examining an organism’s entire DNA, including all its genes. The focus is on the genomes’ structure, function, evolution, mapping and editing.
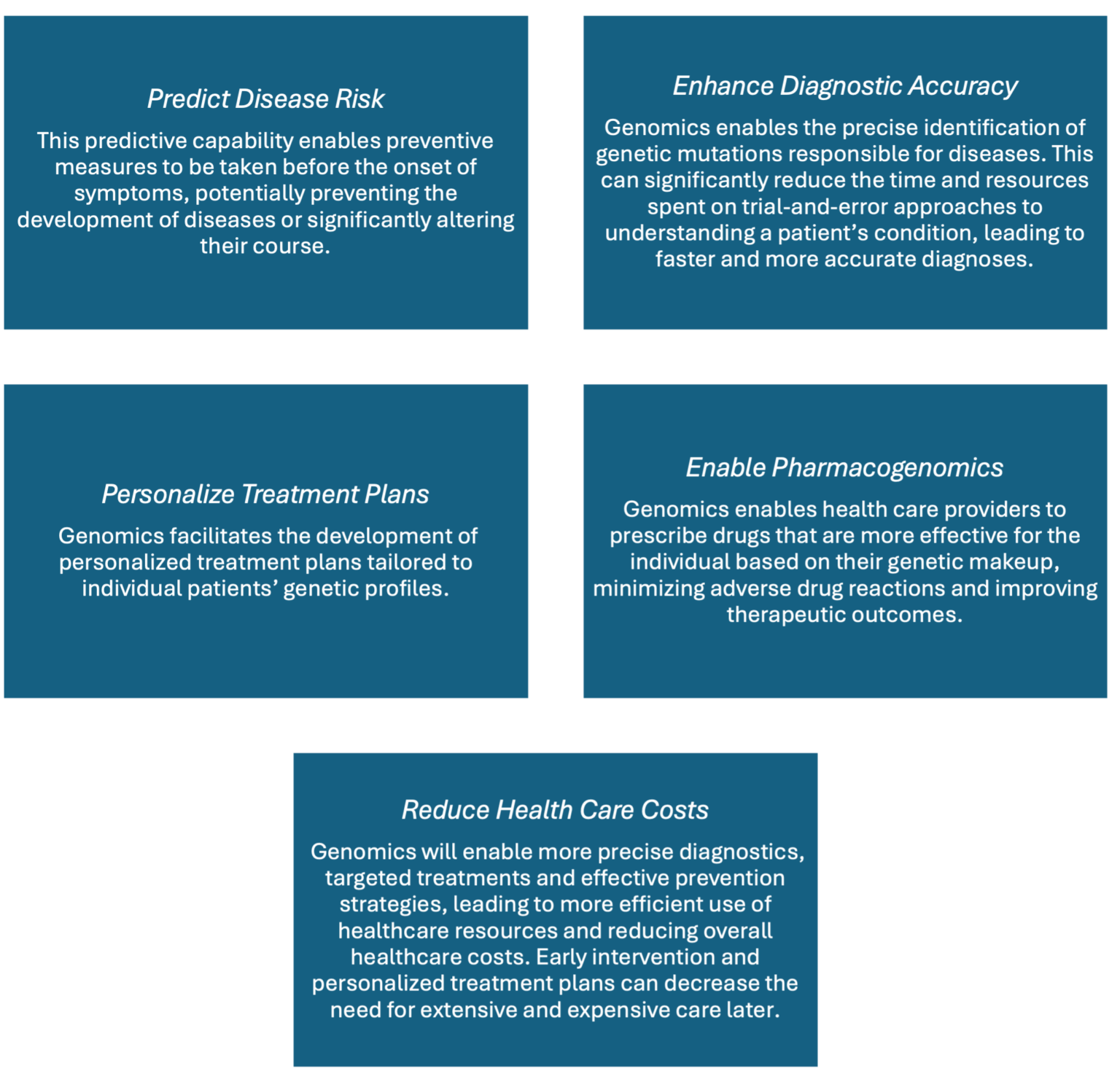
Genomics involves studying genetic sequences to understand genes’ structure, interactions and effects on patients. This process includes analyzing the general characteristics and measurable attributes of all genes, their connections and their collective influence on a patient’s growth and development. The progress in genomics has been driven by the development of technologies that enable fast and cost-effective DNA sequencing and analysis, which is central to transforming medicine. The cost of sequencing the first human genome was $2.7 billion, and it took several international institutes, hundreds of researchers and 13 years to complete. Today, it can be done for $100 (Albert, H., 2024; Pennisi, E., 2022), unlocking the full potential of the human genome. Genomics is poised to significantly improve health care across multiple dimensions, from enhancing diagnostic accuracy to personalizing treatment strategies and preventing and curing diseases. A patient’s medical records will soon routinely include their sequenced genome.

Four innovative technologies—GenAI, big-data analysis, genomics and advanced computing—are concurrently developing to advance precision medicine. We discussed GenAI, big-data analysis and precision medicine in previous articles. Now, let’s examine genomics’ commanding influence on transforming hearing healthcare (HHC).
HOW GENOMICS IS REVOLUTIONIZING HEARING HEALTH CARE
GENE THERAPY

Gene therapy, a medical approach that utilizes genetic material to treat or prevent diseases by correcting the underlying genetic problems, involves modifying a person’s genetic makeup, usually by introducing new genes and repairing or replacing faulty genes within the body’s cells. Gene therapy can be administered in two primary ways: ex vivo (outside the body, where cells are modified and reintroduced into the patient) and in vivo (directly into the body), the preferred method. A new technique, CRISPR, allows us to edit DNA within a cell in vivo. Let’s examine this versatile tool in detail (Hahn & Avrahm, 2023).
GENE EDITING
CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats), the prevailing gene-editing technology, is revolutionizing genetics and health care by providing a powerful tool for precisely manipulating DNA sequences in patients. It offers new possibilities for disease treatment, diagnostics and research. This gene-editing tool is known for its precision, simplicity, and efficiency. It is being applied in various medical fields, including hearing disorders (Xu et al., Z., 2020).
Understanding the Mechanism: The CRISPR-Cas9 system, the backbone of CRISPR technology, is a two-part system. The first part is the Cas9 enzyme, which acts as molecular scissors, cutting DNA at specific points. The second part is a guide RNA (gRNA), which acts as a GPS, directing Cas9 to the exact location in the genome that needs editing. CRISPR can also target multiple genetic mutations simultaneously (Tao, Y. et al., 2023). This unique system enables precise modifications to the DNA of living organisms, allowing for the correction of hearing genetic defects at their source.
CRISPR’s Advantages:
- Precision and Targeted Correction:One key advantage of CRISPR therapy is that it can directly correct the genetic mutations that cause hearing loss at the DNA level. This is a significant departure from traditional treatments, which often only manage symptoms without addressing the underlying genetic cause.
- Permanent Solutions: Unlike hearing aids or cochlear implants, which provide symptomatic relief, CRISPR therapy has the potential to offer a long-lasting, if not permanent, solution to genetic hearing loss by correcting the mutation itself (Fliesler, N., 2019; Chien WW., 2018).
- Broad Application Spectrum: CRISPR’s adaptability allows it to target a wide range of genetic mutations associated with hearing loss, including both recessive and dominant forms of the condition. This broad applicability could make it a viable option for many patients who currently have limited treatment options (Yin et al., 2023; Tao Y. et al., 2023).
- Safety and Specificity: By delivering the CRISPR-Cas9 protein directly into the inner ear cells, researchers have improved the treatment’s specificity, reducing the risk of off-target effects that could arise from more systemic delivery methods. This enhances the safety profile of CRISPR therapy (Gao et al., 2018).
- RNA Editing Capabilities: CRISPR therapy is not limited to DNA editing. CRISPR-Cas13 can target RNA, offering a reversible and potentially safer alternative for modulating gene expression without permanently altering the genome (Zheng Z. et al., 2022; Xiao et al., 2022; Leslie, 2024).
- Overcoming Limitations of Existing Therapies: Current treatments like hearing aids and cochlear implants do not cure hearing loss but rather amplify sound or stimulate the auditory nerve directly to improve hearing perception. CRISPR therapy aims to correct the root genetic cause, potentially restoring natural hearing without the need for external devices.
- Diagnostic Applications: CRISPR technology can be adapted for diagnostic purposes, allowing for the precise identification of genetic mutations that cause hearing loss and leading to earlier and more accurate diagnoses, essential for effective treatment planning and management of hearing disorders (Wu, J. et al., 2023; Yin et al., 2023).
- Innovative Research Tool: CRISPR facilitates the exploration of the genetic landscape of hearing loss, helping to uncover new genes involved in auditory function and disorders. This can lead to discovering novel therapeutic targets and strategies for preventing or reversing hearing loss.
Audiology clinics have limited options to slow down or reverse genetic deafness. Luckily, CRISPR therapy is a major breakthrough in addressing genetic hearing loss, providing accuracy, the possibility of long-lasting correction, flexibility and enhanced safety. As scientific advancements carry on, CRISPR will surely continue to revolutionize the treatment and diagnosis of genetic hearing loss and overcome the drawbacks of current therapies.
Examples of Gene Therapy
Example One: Pioneering animal genetic research. The Spns2 tm1a mouse mutant has a faulty Spns2 gene, which causes it to be unable to maintain the local ionic environment of the inner-ear sensory hair cells. This results in a decreased endocochlear potential, a neurological disorder causing irreversible hearing loss.
In this pioneering research, Martelletti et al. (2023) used genetic intervention to reactivate the faulty Spns2 gene and found they could reverse an existing neurologically based hearing loss.
By activating Spns2 gene transcription at different ages after the onset of hearing loss, they discovered a crucial factor in this therapy. The timing of the therapy was critical. The earlier the activation of the Spns2 gene, the more effective the reversal of the hearing impairment was, underscoring the importance of early intervention for this therapy.
This successful study in mice opens possibilities for future gene therapy for reactivating hearing in people with similar hearing loss. That brings us to example two, the first demonstration of gene therapy for human hearing loss.

Example Two: The first successful hearing-restoring gene therapy for humans. Autosomal recessive deafness 9, caused by mutations of the OTOF gene, is characterized by congenital or prelingual, severe-to-complete, bilateral hearing loss. Before this study, no treatment was available for this congenital deafness.
This single-arm, single-center trial enrolled six children (aged 10 months–18 years) with severe-to-complete hearing loss and confirmed mutations in both alleles of OTOF and without bilateral cochlear implants. A single injection of AAV1-hOTOF was administered into the cochlea through the round window (Jun Lv, et al., 2024).
The gene expression had a time-release pattern. Five of the six children recovered some hearing over time. At 26 weeks after treatment, they showed an average 40–57 dB improvement in the auditory brainstem response (ABR) thresholds at 0·5–4·0 kHz.
The gene therapy technique used in these studies overcomes a significant challenge presented by large genes, such as OTOF, which exceeds the capacity of the widely used adeno-associated virus (AAV) vectors. Researchers addressed this by dividing the OTOF gene into two, encapsulating the halves into separate viruses and injecting a mixture with both halves into the cochlea. This innovative approach allowed the cellular machinery to assemble the complete protein, restoring the cells’ ability to transmit signals to the brain.
The children were cochlear implant candidates, but after acquiring enough hearing from gene therapy, they were no longer eligible for implants. They will be treated with hearing aids. Researchers are following the patients for five years to see if their hearing continues to improve.
Successful OTOF gene therapy treatments are continually emerging, underscoring the importance of pediatric gene therapy. Auditory Insights has published a summary of these studies. For a summary of recent advances in gene therapy for hereditary hearing loss see (Jiang et al., 2023).
Example Three: Genomic Testing for Newborns
A study by Ziegler et al. (2024) has improved our understanding of the feasibility of implementing genome sequencing as an adjunct to traditional newborn screening (NBS) in newborns of different racial and ethnic groups.
Over 11 months, 5555 families were approached, and 4000 (72.0%) consented to participate! Testing was successfully completed for 99.6% of cases. The screen-positive rate was 3.7%, including treatable conditions not currently included in NBS.
DNA sequencing offers an additional method to improve screening for conditions already included in NBS and to add those that cannot be readily screened because no biomarker is currently detectable in dried blood spots. This study encourages us that new parents will welcome newborn genomic screening.
Potential Benefits of Using Gene Therapy to Treat Genetic Disorders in Embryonic Humans
Intrauterine Fetal Gene Therapy
As innovative and promising as the above therapies are, intrauterine fetal gene therapy (IUGT) offers even more promise. Peddi and colleagues (Peddi et al., 2022) have written a comprehensive review that offers an overview of the current knowledge in the field of prenatal gene therapy, as well as potential future research avenues. It is recommended reading. Here are some of intrauterine fetal gene therapy’s distinctive benefits:
- Less Invasive Approach
IUGT provides minimally invasive approaches to preventing genetic disorders by releasing vectors into the embryonic fluids.
- Prevention and Cure of Genetic Disorders
IUGT can prevent or cure genetic diseases by correcting gene mutations in embryos, giving individuals a chance at a healthy life beginning at birth.
- Elimination of Hereditary Disorders
Germ-line gene therapy, which targets reproductive cells, can potentially permanently remove hereditary disorders from a family line.
- Reduction in Health Care Costs
By preventing genetic disorders before they manifest, IUGT could significantly reduce the long-term healthcare costs associated with hospital stays, treatments and lifelong care.
Peddi and colleagues also point out that small fetal size, accessible proliferating progenitor cells, and tolerogenic fetal immune systems make treatment delivery more efficient, safe and long-lasting than after the baby is born.
IUGT provides minimally invasive approaches to preventing genetic disorders. IUGTs are the future of fetal and neonatal medicine to improve quality of life and potentially cure monogenetic disorders before irreversible pathology occurs.
As a result, there are increasing calls to adopt a simultaneous genetic hearing loss screen to the current neonatal health screening guidelines (Luo, h,. 2022). Suppose a NICU AI-based computer vision monitor detects a cerebral dysfunction (Gleason et al., 2024) or a screening test indicates a possible problem—or the parents’ age, family history or medical history puts the baby at increased risk of having a genetic problem. An invasive prenatal diagnostic test and IUGT might be considered in that case. Imagine the benefits of this approach combined with newborn hearing screening to start an otherwise deaf child’s life with hearing.
Ethical and Cultural Considerations
Ninety to 95% of deaf children are born to hearing parents (Mitchell et al., 2004), many of whom would like to make hearing possible for their children. For them, this is a miracle; they can choose gene therapy. However, we must be sensitive to the idea that some deaf parents prefer deaf children and, in both cases, support the parents’ decision. This ethical mindfulness is crucial for ensuring that treatments are developed in a way that respects patient autonomy and diversity (DesGeorges, J., 2016).
Resulting Patient Demographic Shifts
Most therapies work best when they are started as early as possible. The gene therapy examples discussed above confirm that gene therapies also conform to this “earlier is better” rule. In addition to the IUGT advantages listed above, it is the earliest possible therapy. As a result, we can expect an increase in the number of pediatric and fetal patients receiving treatment.
Currently, newborn hearing screening demands a small cadre of pediatric audiologists.Most audiologists treat seniors, where the demand is greatest.Still, because gene therapies work best in younger patients, we must plan for an increased emphasis on pediatric patients and inter-uterine fetal gene therapies. To the extent gene therapy is successful, IUGT patients may experience a lifetime without congenital sensorineural hearing loss, reducing the demand from older patients.
Traditional Audiology Still Has a Role

Gene therapy will not entirely replace hearing aids and cochlear implants—at least not for a generation or two. Because gene therapy works best at younger ages, the boomers will still need and expect traditional therapies while we shift toward younger genome therapy patients. The baby boomer generation’s peak is reaching 65 years of age—4.1 million Americans are reaching age 65 annually. Many of those at 65 still have 10, 20, or even 30 years ahead as life expectancy increases. Additionally, the second half of this large boomer generation is still under 65.
Gene therapies are still in their infancy and need time to be perfected. Gene therapy has shown that it is not always a complete solution or cure for humans (Jun Lv et al., 2024) and animals (Martelletti et al., 2023). For instance, in the case of OTO-F-treated children, while they no longer required cochlear implants, they still needed hearing aids. This highlights the imperfections of gene therapy. Not all gene therapies will immediately work perfectly or last a lifetime. This is when the collaboration between audiologists and geneticists becomes crucial. It is our future! Together, we must devise successful new treatments for patients who have undergone gene therapy. Some of these treatments will be traditional, some will be modifications of traditional treatments and some will be innovative solutions that we can’t yet envision.
Because of the rapid development and success of gene therapy, we surely must now plan for the shift of our expertise to younger patients and a growing partnership with genomics. Those forces will dominate the future for today’s and tomorrow’s audiology students. To allow us to make this shift, we must use audiology extenders and AI to care for nonprescription patients and to free us to work with geneticists to treat more complicated patients requiring prescription care. Audiologists who participate in precision medicine and actively collaborate with geneticists will thrive.
Soon, audiologists will work in a healthcare world based on precision medicine, utilizing advanced machine intelligence on powerful computers, personalized by genomics and massive data sets. Prepare yourself!
Resources for Future Study


References
Albert, H., (2024). In Conversation with Gilad Almogy. Inside Precision Medicine, April 2024, pp. 28–30.
Aronson, S., Rehm, H. (2015). Building the foundation for genomics in precision medicine. Nature 526, 336–342. https://doi.org/10.1038/nature15816.
Chien WW. (2018). A CRISPR Way to Restore Hearing. N Engl J Med. 2018 Mar 29;378(13):1255-1256. doi: 10.1056/NEJMcibr1716789. PMID: 29601257; PMCID: PMC6698387.
DesGeorges, J., (2016). Avoiding Assumptions: Communication Decisions Made by Hearing Parents of Deaf Children, AMA J Ethics. 2016;18(4):442-446. doi: 10.1001/journalofethics .2016.18.4.sect1-1604
Fliesler, N., (2019). Optimized CRISPR/Cas9 gene editing averts hearing loss in ‘Beethoven’ mice, Boston Children’s Hospital Post, 2019, July 3.
Gao, X., Tao, Y., Lamas, V. et al. (2018). Treatment of autosomal dominant hearing loss by in vivo delivery of genome editing agents. Nature 553, 217–221 https://doi.org/10.1038/nature25164
Gleason, A., (2024). Detection of neurologic changes in critically ill infants using deep learning on video data: a retrospective single center cohort study. The Lancet, 2024 (in press) www.thelancet.com Open access.
Hahn & Avrahm (2023). Gene Therapy for Inherited Hearing Loss: Update and Remaining Challenges, Audio Res, 13, 952-966.
Jiang, L. et al., 2023 Advances in gene therapy hold promise for treating hereditary hearing loss. Molecular Therapy, Vol. 31 No4, April 2023. Open Access.
Jun Lv, et al. 2024, AAV1-hOTOF gene therapy for autosomal recessive deafness 9: a single-arm trial, Lancet Online First, January 24, 2024 DOI: https://doi.org/10.1016/S0140-6736(23)02874-X https://auditoryinsight.com/wp-content/uploads/securepdfs/2024/02/Gene-Therapy-Clinical-Trials-Results.pdf
Leslie, M. (2024) EDIT KILL THE MESSENGER: New treatments aim to counteract mutant genes by fixing the faulty RNA they produce, Science, Vol. 386, issue 6720, doi: 10.1126/science.z5vhbw1
Luo, H., Yang, Y., Wang, X., Xu, F., Huang, C., Liu, D., Zhang, L., Huang, T., Ma, P., Lu, Q., Huang, S., Yang, B., Zou, Y., & Liu, Y. (2022). Concurrent newborn hearing and genetic screening of common hearing loss variants with bloodspot-based targeted next generation sequencing in Jiangxi province. Frontiers in Pediatrics, 10, 1020519. https://doi.org/10.3389/fped.2022.1020519
Martelletti, E., Ingham, N., Steel, K., (2023). Reversal of an existing hearing loss by gene activation in Spns2 mutant mice, PNAS, Vol. 120 No.34 e2307355120; https://www.pnas.org/doi/10.1073/pnas.2307355120
Mitchell, R.E., & Karchmer, M.A. (2004). Chasing the Mythical Ten Percent: Parental Hearing Status of Deaf and Hard of Hearing Students in the United States. Sign Language Studies 4(2), 138-163. https://doi.org/10.1353/sls.2004.0005
Peddi NC, Marasandra Ramesh H, Gude SS, Gude SS, Vuppalapati S. Intrauterine Fetal Gene Therapy: Is That the Future and Is That Future Now? Cureus. 2022 Feb 23;14(2):e22521. doi: 10.7759/cureus.22521. PMID: 35371822; PMCID: PMC8951626.
Pennisi, E., (2022). A $100 genome? New DNA sequencers could be a ‘game changer’ for biology, medicine. Science News,15 JUN. doi: 10.1126/science.add5060
Tao, Y., Lamas, V., Du, W. et al. Treatment of monogenic and digenic dominant genetic hearing loss by CRISPR-Cas9 ribonucleoprotein delivery in vivo. Nat Commun 14, 4928 (2023). https://doi.org/10.1038/s41467-023-40476-7
Wu, J., Tao, Y., Deng, D. et al. The applications of CRISPR/Cas-mediated genome editing in genetic hearing loss. Cell Biosci 13, 93 (2023). https://doi.org/10.1186/s13578-023-01021-7
Xiao, Q., et al., (2022). Rescue of autosomal dominant hearing loss by in vivo delivery of mini dCas 13X-derived RNA base editor. Sci. Transl. Med. 14, eabn0449 (20 July,2022)
Xu et al., Z., 2020
Yin, G., Wang, H., & Sun, Y. (2023). Recent advances in CRISPR-Cas system for the treatment of genetic hearing loss. American Journal of Stem Cells, 12(3), 37-50. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10509501/
Zheng, Z., Li, G., Cui, C., Wang, F., Wang, X., Xu, Z., Guo, H., Chen, Y., Tang, H., Wang, D., Huang, M., Chen, Z., Huang, X., Li, H., Li, G., Hu, X., & Shu, Y. (2022). Preventing autosomal-dominant hearing loss in Bth mice with CRISPR/CasRx-based RNA editing. Signal Transduction and Targeted Therapy, 7(1), 1-13. https://doi.org/10.1038/s41392-022-00893-4